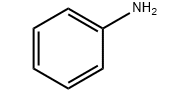
Lebitso la Sehlahiswa:Aniline
Sebopeho sa molek'hule:C6H7N
Nomoro ea CAS:62-53-3
Sehlahisoa sebopeho sa molek'hule:
Lintho tsa Lik'hemik'hale:
Aniline ke amine ea mantlha e nkhang hamonate le motsoako o entsoeng ka ho nkela athomo ea haedrojene sebakeng sa molek'hule ea benzene ka sehlopha sa amino. Ke oli e se nang mebala e kang mokelikeli o tukang o monko o matla. Ha e futhumetse ho 370 C, e qhibiliha hanyane ka metsing 'me e qhibiliha ka har'a ethanol, ether, chloroform le lihlapolli tse ling tsa tlhaho. E fetoha sootho moeeng kapa tlas'a letsatsi. E ka tšeloa ka mouoane. Ho eketsoa phofo e nyane ea zinki ho thibela oxidation ha e silafatsoa. Aniline e hloekisitsoeng e ka eketsoa 10 ~ 15ppm NaBH4 ho thibela ho senyeha ha oxidation. Tharollo ea aniline ke alkaline.
Ho bonolo ho hlahisa letsoai ha le kopana le asiti. Liathomo tsa haedrojene tse lihlopha tsa eona tsa amino li ka nkeloa sebaka ke lihlopha tsa alkyl kapa acyl ho hlahisa aniline ea bobeli kapa ea boraro le acyl aniline. Ha karabelo ea phetoho e etsahala, lihlahisoa tsa ortho le para substituted lihlahisoa li hlahisoa haholo-holo. E kopana le nitrite ho etsa matsoai a diazonium, a ka sebelisoang ho hlahisa letoto la metsoako ea benzene le metsoako ea azo.
Kopo:
Aniline ke e 'ngoe ea li-intermediate tsa bohlokoa ka ho fetisisa indastering ea dae. E ka sebelisoa indastering ea dae ho etsa enke ea acid e putsoa G, acid medium BS, acid soft yellow, direct orange S, direct rosé, indigo blue, dissese yellow brown, cationic rosé FG le reactive brilliant red X-SB, joalo-joalo; ka pigments organic, e sebelisoa ho etsa khauta khubelu, khauta khubelu g, khōlō khubelu phofo, phenocyanine khubelu, oli qhibilihang ka har'a ba batsho, joalo-joalo E ka boela ea sebelisoa e le lintho tse bonahalang tse tala bakeng sa meriana sulfa lithethefatsi, 'me e le ea mahareng tlhahiso ea linoko, polasetiki, varnishes, lifilimi, joalo-joalo E ka boela ea sebelisoa e le explossionproof le an a stabilizer, a explososproof, an gastroenter solvent; e ka boela ea sebelisoa ho etsa hydroquinone le 2-phenylindole.
Aniline ke thepa e tala ea bohlokoa bakeng sa tlhahiso ea chefo e bolaeang likokoanyana.
Lihlopha tsa lihlahisoa
-

Mohala
-

E-mail
-

Whatsapp
-

Holimo













